In May 2016, the World Health Assembly endorsed the Global Health Sector Strategy (GHSS) on viral hepatitis 2016–2021. The GHSS calls for the elimination of viral hepatitis as a public health threat by 2030 (reducing new infections by 90% and mortality by 65%).
Most of the burden of disease from hepatitis B virus (HBV) infection comes from infections acquired before the age of 5 years. Therefore, prevention of HBV infection focuses on children under 5 years of age. The United Nations selected the cumulative incidence of chronic HBV infection at 5 years of age as an indicator of the Sustainable Development Goal target for “combating hepatitis” . This indicator is measured indirectly through the proportion of children 5 years of age who have developed chronic HBV infection (i.e. the proportion that tests positive for a marker of infection called hepatitis B surface antigen [HBsAg]).
The strategy includes both impact (incidence and mortality) and service coverage target. By 2020, five million people will be receiving treatment for chronic hepatitis B virus infection and the number of new cases of chronic hepatitis infection would have been reduced by 30% compared with the number of new cases in 2015. By 2030, the incidence of chronic hepatitis infection will have been reduced by 90% and there will be universal access to key prevention and treatment services.
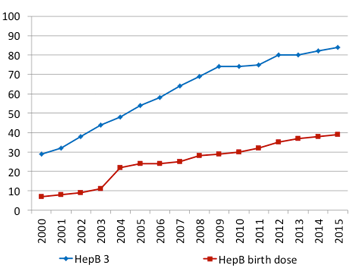
An early win in the global response to viral hepatitis was achieved through the effective scaling up of hepatitis B vaccine. WHO recommends that all infants receive the hepatitis B vaccine as soon as possible after birth, preferably within 24 hours. Birth-dose vaccination is a key intervention for prevention of hepatitis B virus infection in infants. However, its delivery can be a challenge in communities where a large proportion of births occur outside of health facilities. As a result, global coverage is only around 38% In 2015, global coverage with the three doses of hepatitis B vaccine in infancy reached 84%. This has substantially reduced HBV transmission in the first five years of life, as reflected by the reduction in HBV prevalence among children to 1.3%. Other prevention interventions are available but insufficiently implemented.
Global Health Sector Strategy on Viral Hepatitis 2016 – 2017
WHO recommends that all infants receive the hepatitis B vaccine as soon as possible after birth, preferably within 24 hours. If administration within 24 hours is not feasible, a late birth dose has some effectiveness. Although effectiveness declines progressively in the days after birth, after 7 days, a late birth dose can still be effective in preventing horizontal transmission and therefore remains beneficial. WHO recommends that all infants receive the late birth dose during the first contact with health-care providers at any time up to the time of the next dose of the primary schedule.
The birth dose should be followed by 2 or 3 doses to complete the primary series. In most cases, 1 of the following 2 options is considered appropriate:
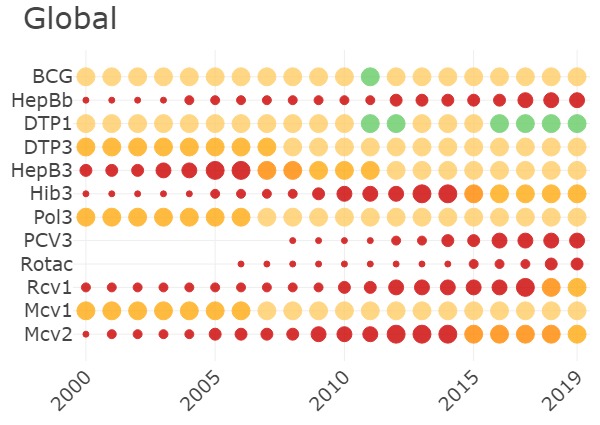
This dashboard shows the HBsAg prevalence estimates at global, regional and country level and how they have changed since hepatitis B vaccination was introduced.
This dataset represents the best estimates for the hepatitis B surface antigen indicator and aims to facilitate comparability across countries and over time. The estimates are not always the same as the official national estimates, because of the use of different methodologies and data sources. Estimates are provided for 194 WHO Member States. The analysis was carried out for the age groups 0-5 years and for the general population. Due to scarcity of data from some countries, the estimates are more robust at global and regional level than at country level, therefore, we suggest countries focus on the 95% Credible Intervals and not only on the reported point estimates.
WHO’s estimates uses a methodology reviewed by the Immunization and Vaccines Related Implementation Research Advisory Committee (IVIR-AC) and presented to the Strategic Advisory Group of Experts (SAGE). These estimates have been documented following the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER). More detailed information on quality of data sources and methods, as well as estimated uncertainty intervals, is provided in the Global Hepatitis Report 2017, the WHO Immunization surveillance, assessment and monitoring system, the estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2017, and in other referenced sources.
WHO provided Member States the opportunity to review and comment on data and estimates as part of the so called country consultation process.
The database last update was 23 March 2017. Estimates will be updated as more recent or revised data become available, or when there are changes to the methodology being used. Next scheduled update will be in Q1 2018. Member States, civil society, country and regional offices that wish to contribute to improve the seroprevalence database can send their potential eligible published and unpublished reports on surface antigen prevalence to the following email: VaccineResearch@who.int until October 15, 2017. They will be screened according to the inclusion criteria of the review. Potential contributions received after this date will be considered in the next update exercise.
Hepatitis b surface antigen estimates and number of carriers in 2015 in the general population.
Hepatitis b surface antigen estimates and number of carriers in 2015 in the under 5 years of age.
Hepatitis b surface antigen estimates and number of carriers in 2020 in the general population.
Hepatitis b surface antigen estimates and number of carriers in 2020 in the under 5 years of age.

Disclaimer: The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. © WHO 2016. All rights reserved.
HBsAg estimates, modelling conducted by the London School of Hygiene and Tropical Medicine.
|
Total Population
|
|
|
Total Population under 5
|
|
|
Births
|
|
|
Infant Mortality
|
|
|
Urban Population
|
|
|
Births attended by skilled health personnel *
|
% |
Source: United Nations, Population Division. The World Population Prospects - the 2017 revision". New York, 2017
Note: Births and Infant Mortality refers to five-year periods running from 1 July to 30 June of the initial and final years.
* Births attended by skilled health personnel is from WHO Health Statistics 2017. Data are scarce, comes from different years, and are not available for every country.
|
HepB vaccine introduced nationwide
|
|
|
HepB birth dose introduced
|
|
|
Current schedule
|
|
|
HepB vaccine types
|
Source: http://www.who.int/immunization/monitoring_surveillance/data/en/
Source: http://www.who.int/immunization/monitoring_surveillance/data/en/
| Level | Author, Date | Years | Ages | pHBsAg | Low CI (for HBsAg) | Hi CI (for HBsAg) | Country | ISO3 | Region | IAA | Link to report |
|---|
We present national-level prevalence estimates of chronic HBV derived by a systematic review of peer-reviewed literature reporting HBV prevalence (hepatitis B surface antigen [HBsAg]) in children under 5 years of age and the general population. We updated the systematic review conducted by Schweitzer, et al., 2015. It included a systematic search on articles published between Jan 1, 1965, and Oct 23, 2013. We updated the systematic search on articles published between Oct 23, 2013, and March 20, 2017 and searched the databases Embase, PubMed, Global Index Medicus, Popline, and Web of Science. The inclusion/exclusion criteria were similar to (Schweitzer, et al.). Observational studies on chronic HBV infection seroprevalence (HBsAg prevalence), done in the general population or among blood donors, health-care workers (HCWs), and pregnant women were considered for inclusion in this systematic review. Studies were if they were systematic reviews or meta-analyses, surveillance reports, case studies, letters or correspondence, or did not contain HBsAg seroprevalence data. Studies were also excluded if they exclusively reported prevalence estimates for high-risk population groups (e.g., migrants and refugees).
The data was modelled using a logistic regression, weighting each study by its size and using a conditional autoregressive model accounting for spatial and economic correlations between similar countries. The response variable in the model was the prevalence of Hepatitis surface antigen (HBsAg) with the predictor variables being age (three categories, under 5, juvenile (5-15) and adult (16+), split using the average age of participants in the study), sex (proportion female in the study), study bias (e.g. a high fraction of study participants from indigenous populations), 3 dose vaccine coverage, birth dose of the vaccine and country of study.
The model was simulated in the Bayesian statistical package WinBUGS, and data manipulation and model initialisation run from R (3.3.1) using R2WinBUGS. We considered the parameters of age, sex, study bias (e.g. a high fraction of study participants from indigenous populations), vaccine coverage, birth dose of the vaccine and country of study. The coverage of routine 3 dose vaccination and birth dose vaccination was calculated by cross referencing the year of and age of participants in each study with the corresponding WHO-UNICEF vaccine coverage estimates for that country.
We used the CAR-normal function, in WinBUGS, to model the spatial and developmental autocorrelation related to neighbouring countries. For each country for which we had prevalence data a weighted central position was calculated using the size and location of each study. For those with no data, we used the population centroid. In a novel approach, we considered 3 dimensions in the country proximity matrix; we used the usual geographic dimensions, latitude and longitude and also combined these with the natural log of the country’s GDP per capita.
 Acknowledgements
to the London School of Hygiene and Tropical Medicine who conducted the modelling of the
hepatitis
B surface antigen estimates.
Acknowledgements
to the London School of Hygiene and Tropical Medicine who conducted the modelling of the
hepatitis
B surface antigen estimates.